Abstract
Introduction: Multiple myeloma (MM) is an incurable plasma cell neoplasm. Despite several effective frontline therapeutic regimens, including Bortezomib (BTZ), the relapse is almost inevitable; therefore, better therapeutic modalities to improve the outcomes are needed. Cyclin-dependent kinases (CDKs) are an essential constituent of the cellular transcriptional machinery. Many tumor types, including MM are critically dependent on transcription to maintain their oncogenic state. Therefore, pharmacological modulation of CDK7 kinase activity is considered an attractive approach to treating MM. In this study, we evaluated anti-myeloma activity and functional consequences of CDK7 inhibition by a covalent inhibitor of CDK7 (THZ1) using BTZ-resistant in vitro, ex vivo, and zebrafish xenograft models of multiple myeloma.
Materials and methods: Primary human CD138+ cells were isolated using Magnetic Activated Cell Sorting (MACS) from bone marrow samples collected at diagnosis and at the time of relapse of MM patients. All patients received BTZ based therapy at diagnosis. In house developed paired BTZ sensitive (BTZS) and resistant (BTZR) H929 cells, MM lines such as RPMI8226, U266 and normal human immortalized B lymphocytes, and bone marrow stromal HS5 cells (BMSC) were exposed to varying concentrations of THZ1 alone or with BTZ. Following treatment of MM cells, in vitro assays of cell cycle, cell proliferation, apoptosis, NF-κB signalling based immune blots and co-culture (BMSC with MM) experiments were performed. The effects of CDK7 inhibition on tumor regression were examined using MM zebrafish xenograft models.
Statistical analyses: GraphPad Prism software V.8 was used. Data points were expressed as mean ± SD of triplicate experiments. The significance of differences between experimental variables was determined using the student t-test or one-way ANOVA with Tukey post-test.
Results: THZ1 potently reduces multiple myeloma cell proliferation of BTZR cells (IC50:0.09µM) and primary MACS sorted CD138+ plasma cells from de novo (n=21 mean IC50:0.04µM; range 0.02-0.3µM) and BTZ treated relapsed/refractory MM pts (n=5; mean IC50:0.06µM; range 0.01-0.5µM). THZ1 suppresses the phosphorylation at Ser 2,5 & 7 of carboxy terminal domain of RNA polymerase II in and downregulates the transcription of BCL2 family of proteins both in BTZS & BTZR cells. THZ1 alone or in combination with BTZ markedly diminished MM cell survival in BTZR cells by upregulating cleaved PARP and cleaved caspase-3 and downregulating MCL-1, BCL-XL and survivin protein levels in time and dose-dependent manner. The co-culture of MM cells with bone marrow stromal cells in the presence of THZ1 sensitizes the BTZ-resistant cells towards apoptosis. THZ1 was also found effective in eliminating the primary CD138+ plasma cells from relapse/refractory patients who were previously treated with BTZ based therapy.
Cyclin D1 expression was significantly reduced at 8h and 24h of THZ1 treatment. In time-dependent experiment, THZ1 also increased the stability of P53 in MM cells. Furthermore, cell cycle distribution assessment revealed that THZ1 induced G1/S phase arrest in BTZS and BTZR cells. These effects were associated with diminished T160 CDK2 phosphorylation and S807 Rb phosphorylation.
Furthermore, THZ1 treatment distinctly suppressed the TNF-α stimulated IKK phosphorylation of IκBα & NF-κB-p65 in BTZS & BTZR cells, with no evident impact on total IκBα and NF-κB-p65 protein levels. Activation of IKKα tends to phosphorylate IκBα at S32, which triggers IκBα degradation in the proteasome resulting in rapid nuclear translocation of NF-κB complex. The p-IKKα/β, cFLIP, CFLAR and REL A levels were also decreased following THZ1 treatment.
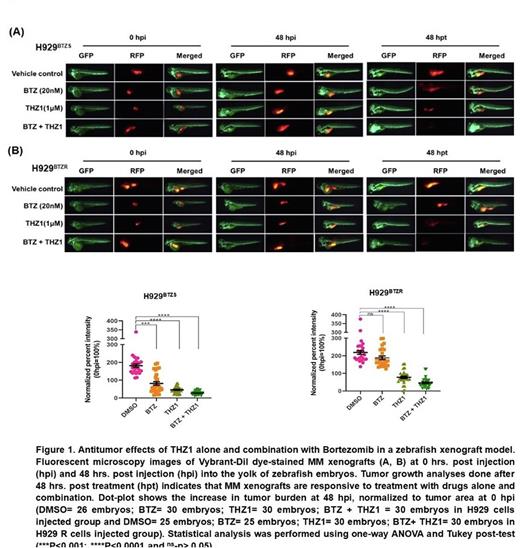
To validate in vitro and ex vivo findings, fluorescently labeled BTZS & BTZR cells were injected in the yolk area of zebrafish embryo. After 48 hpi the fluorescent intensity was increased 59% and 65% in xenotransplanted embryos compared with 0 hpi. However, BTZS cells engrafted zebrafish embryo treated with THZ1 or BTZ alone for 48 hpt significantly decreases the tumor growth compared with 0 hpi and 48 hpi. BTZR cells injected embryo did not respond to BTZ, but THZ1 alone or in combination significantly reduced the tumor growth (figure 1).
Conclusion: We have shown that THZ1 can overcome Bortezomib resistance and induces apoptosis by inhibiting canonical NF-kβ signaling in multiple myeloma.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal